Abstract
Introduction: Chimeric antigen receptor T-cell (CAR-T) therapy has achieved remarkable efficacy in B-cell acute lymphoblastic leukemia (B-ALL). However, relapse after CAR-T has been a major issue. CAR T-cells target multi-antigen or CAR-T combined with other strategy may reduce the relapse post CAR-T. The main purpose of this study is to observe the safety and efficacy of CD22/CD19 CAR-T bridging to autologous stem cell transplantation (auto-HSCT) in B-ALL patients.
Methods: A total of 12 newly diagnosed B-ALL patients were enrolled in this study (NCT05470777). These patients received induction and consolidation chemotherapy according to standard protocols of our institute. Autologous lymphocytes were collected at week 4 after induction chemotherapy. Afterwards, autologous CAR T-cells targeting CD22 and CD19 (co-stimulatory molecule was 4-1BB and infusion dose was 5×106/kg, respectively) were sequential infused after lymphodepletion chemotherapy with fludarabine and cytophosphamide. Autologous stem cells mobilization and collection were performed at 6-8 weeks after CAR T-cells infusion. After conditioned with modified BuCy, autologous stem cells were infused. No maintainence therapy was performed after auto-HSCT. Minimal detectable disease (MRD) was monitored by flow cytometry. The median follow-up was 17.5 months (range, 5 - 27 months). Persistence of CAR T-cells were detected using real time quantitative RT-PCR (qPCR).
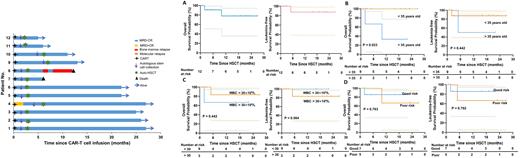
Results: A total of 12 patients were enrolled. Nine (9/12, 75%) were Ph negative B-ALL, 2 (2/12,16.7%) were Ph positive B-ALL, and 1 (1/12, 8.3%) were Ph-like B-ALL. 7 (58.3%) patients had poor risk factors according to NCCN guidelines. Two (16.7%) patients developed grade 1-2 cytokine release syndrome (CRS). No grade 3-4 CRS and immune effector cell associated neurotoxicity syndrome (ICANS) occurred. After CAR-T therapy, all patients achieved complete remission (CR), with 11 MRDnegativeCR (MRD-CR) and 1 MRDpositiveCR (MRD+CR). Until the last follow-up on June 1st, 2022, 83.3% of enrolled patients remain MRD-CR. Duration of continuous MRD-CR was detected in 4 patients for more than 2 years and in 7 patients for more than 1 year. The median OS and LFS in all 12 patients are not reached. One (1/12, 8.3%) patient relapsed. This patient developed antigen-negative relapse at 7 months after auto-HSCT. She then underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) but died of progressive disease at 2 months after allo-HSCT. One patient died at 2 months after auto-HSCT due to pulmonary infection. Amplification of CAR-T cells were detected in all patients in the follow-up (range, 1-24 months). Interestingly, CAR-T cells were detected in autologous stem cells from 3 patients and were still detected in the peripheral blood samples in 4 patients within one week after autologous stem cells infusion.
Conclusions: Our preliminary study demonstrated that autologous CD22 and CD19 CAR-T cell infusion bridging auto-HSCT as a consolidation strategy showed favorable safety and efficacy in B-ALL. The harvest of autologous stem cells after CAR-T cell therapy allows the persistence of CAR-T cells in the stem cells, which may explain the persistence of CAR-T cells after autologous transplantation despite the myeloablative conditioning regimen used in our study.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal